Filteredwaterguide.com is supported by readers. If you purchase through referral links on our site, we make a commission at no extra cost to you. Learn more.
In this blog, we go over the key differences for salt-free and salt-based water softeners and go over these key takeaways:

Both salt free “softeners” and salt based softeners are pretreatment equipment for water. That’s about the only thing they share in common in terms of their operational purpose.
While the mechanics behind each one’s “softening” process varies bigtime, both are intended to minimize the negative impact of calcium hardness scale within your home’s appliances, piping and glass shower doors.
Over the years, companies have been somewhat misleading by using the term salt-free softeners. I’m sure they argue that using the term “softener” when describing their salt free product is just a matter of semantics. In reality, a salt free softener and a salt based softener are both quite different.
The term “soft water” is water containing less than one grain per gallon of calcium. For those unaware of what a grain of calcium is, 1 grain of calcium is equal to 17.1 mg/L (milligrams per liter). In empirical measurements, 17.1 mg/L is the same as saying 17.1 pounds of calcium per million gallons of water.
The meaning of soft water is important to highlight because a water softener, by definition, would function as a device that’s able to produce water that has a concentration of calcium less than 1 grain or 17.1 mg/L or ppm.
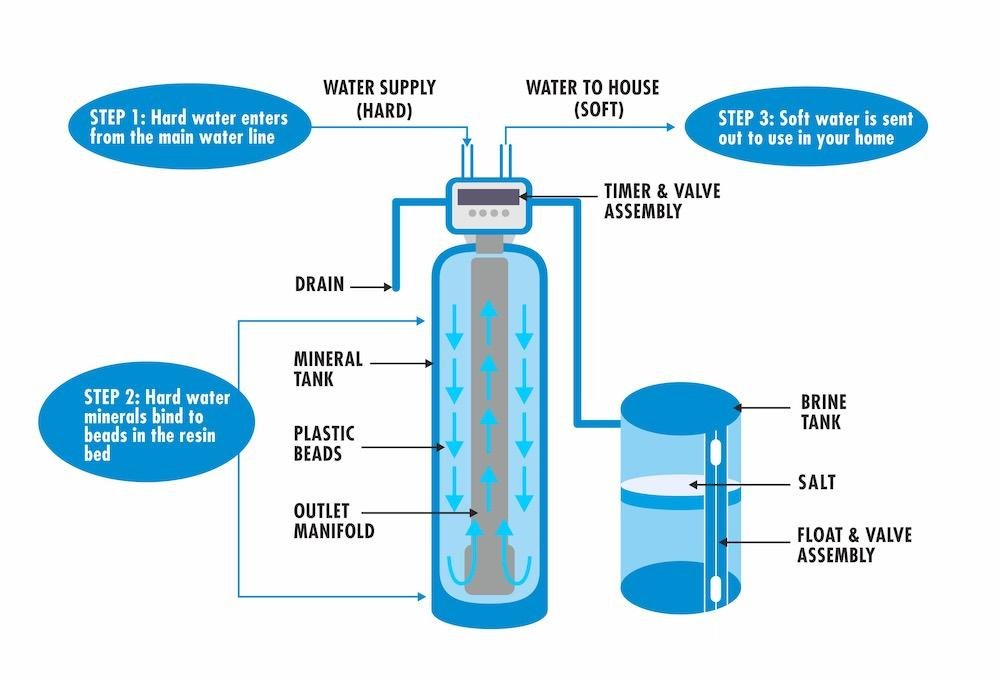
A salt based water softener utilizes a process called ion exchange to remove calcium and magnesium from a water source. Inside a water softener, a bed of cationic resin is present.
If you were to cut open a water softener tank, in the bottom you would see tiny orange beads about the size of the ball in a ball point pen. Under a microscope, the resin beads look like tiny balls of yarn, which increases their surface area so more ions can cling to them.
As water passes through the resin, calcium and magnesium are exchanged for sodium ions and the effluent water is now by definition soft.
With a salt based softener system, once the resin bed is saturated with calcium, regenerating the resin with concentrated sodium or potassium chloride brine water is required to effectively scrub the calcium ions from the resin surfaces before being placed back into service.
On the other hand we have salt free softeners, which I have already mentioned is misleading because they don’t actually remove calcium or magnesium from the effluent water at all.
In fact, there’s the same amount of both calcium and magnesium entering a salt free water softener as there is leaving it. Within salt based softener system tanks lies a bed of polymer beads, similar to shape and size as the resin found in salt based softeners.
Under a microscope rather than appearing like a ball of yarn, their polymer beads look like a pickleball. As calcium and magnesium enter the bed of polymer beads, the calcium and magnesium ions are attracted to the polymer bead.
As water continues to enter the polymer bed, the calcium and magnesium build up on the bead’s exterior to the point both ions crystallize.
The newly formed calcium and magnesium crystals then detach from the polymer bead and exits the salt free softener. At no point during a salt free softener’s operation does it require regeneration.
| Purpose | Salt-Free Conditioner | Salt Based Softener |
|---|---|---|
| Health and Beauty Benefits | X | |
| Environmental Concerns | X | |
| Low Equipment Maintenance Upkeep | X | |
| Reduced Cost of Ownership | X | |
| Spotless Dishes/Appliances | X | |
| Low Energy Demand | X | |
| Easy Installation | X | |
| Total Calcium and Magnesium Removal | X | |
| Reduced Deposit Cleaning | X | X |
| Car Washing | X | |
| RO System Pretreatment | X | |
| Household W/ Primarily Copper or Metal Piping | X | |
| Reduced Soap and Detergent Usage | X |
Each piece of equipment serves a purpose. For salt based softener systems, you get the entire package with the water quality it produces, with having to add salt being the most noted inconvenience. The frequency of the need to add salt can be overcome through increasing the size of the brine tank, so this is an issue that can be prevented ahead of time as long as there is room for a larger tank. This type of system may be a no-brainer for individuals that want it all and are not concerned with the cost or labor associated with occasionally adding a bag or two of salt to the brine tank each month. If you are only interested in delaying the formation of calcium carbonate in your plumbing and appliances and potentially ease the elbow grease required to clean calcium carbonate scale then a salt free system might be right for you. Think about what you are really looking for, weight the pros and cons and see what’s right for you!
© 2024 Filtered Water Guide. All rights reserved.