Filteredwaterguide.com is supported by readers. If you purchase through referral links on our site, we make a commission at no extra cost to you. Learn more.
There’s a whirlwind of options out there for softeners, and one option coming to the forefront is having a sodium softener versus a potassium softener. We’ll we’re here to answer some of the most common questions we are asked to hopefully help you on your quest to find the best suited softener for your needs.
The ability for potassium chloride versus sodium chloride to soften water has nothing to with the type of salt you use, it’s actually the resin that enables water to be softened.
Selecting one over the other has to do with what you are trying to accomplish, and what the system is being used for. In your situation the choice may end up being a no brainer after reading this article.

Potassium chloride as well as sodium chloride are classified as inorganic metal alkali salts that can both be used to regenerate resin within water softener vessels to create water that is absent of calcium ie. soft water.
Inside all ion exchange tanks is what’s called media. In a water softener vessel specifically, there’s cationic resin. Cationic resin in general looks like tiny beads with an appearance very similar to masago (the little orange fish eggs found on sushi).
Under a microscope the resin looks like a ball of twine, which increases the surface area of the resin allowing it to hold onto the greatest amount of either sodium, calcium or potassium ions as possible.
When water passes through the softener’s resin bed the calcium (ionic charge of +2) has a higher ionic charge than sodium (ionic charge of +1).
Because calcium has a stronger ionic charge than both sodium and potassium, the weaker sodium or potassium ion is exchange by the resin and is carried away in the now soft water discharge or effluent.
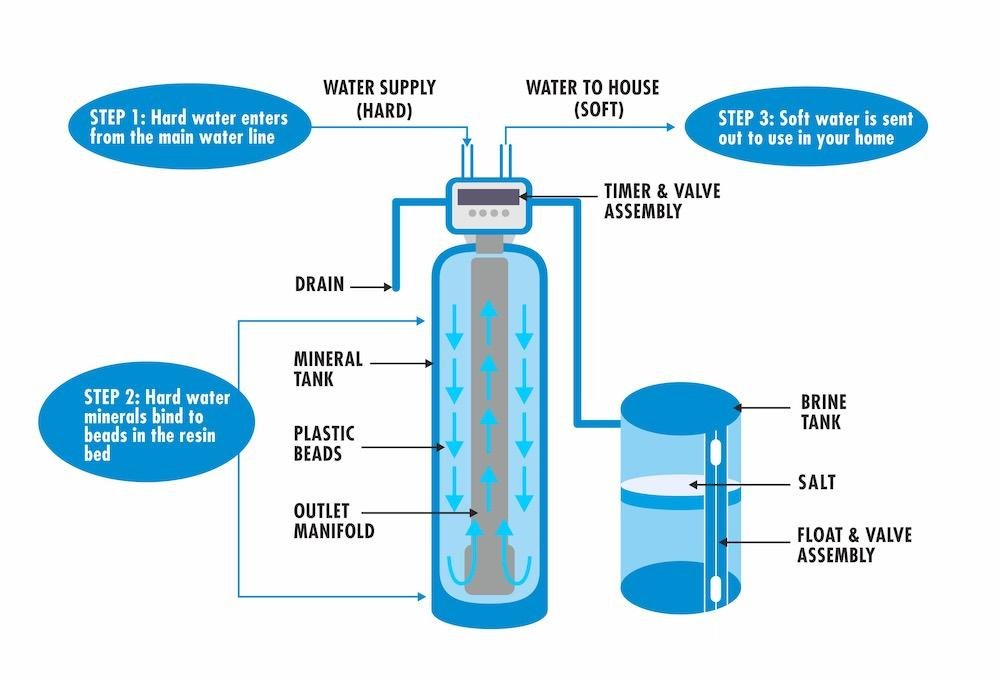
Once the softener’s resin bed is completely saturated with calcium ions, the softener has now reached its capacity and is now in need of regeneration.
There are four stages to softener regenerations. The first stage of regeneration is the backwash, where fresh water is flowed in reverse through the softener resin bed, allowing the compacted resin bed to fluff up.
In the second stage, either sodium chloride or potassium chloride brine is flowed through the systems media at concentrated levels which displaces the calcium from the resin and carries it away with the residual concentrated sodium chloride and or potassium chloride and sent to the drain.
The third process is the slow rinse, where water is forced through the resin bed in the normal operating direction to begin the purge of brine water. And the fourth and final stage is the fast rinse, where water is pushed through the softener at high cubic feet per second to re-compact the resin and to remove any residual brine from the system before the unit goes back into service.
Due to the fact that during the ion exchange process of soft water production either sodium or potassium will be introduced into the soft water effluent there are some things that should be taken into consideration to promote wellbeing.
For most people, the amount of sodium or potassium a properly set and functioning softener adds to the water is negligible and cannot be detected. Nevertheless, if you can stay away from something you need to, why take the risk especially when it comes to your health.
For individuals with restrictions on their allowable sodium intake, utilizing sodium chloride based softeners might create a situation where you are unintentionally consuming sodium and leading to adverse health effects. In this case, it may be best to opt for potassium chloride based salt for your softener operation.
Running your softener system with Potassium Chloride is more expensive opposed to utilizing Sodium Chloride.
#1. With potassium chloride, you will have approximately a 10% increase in the amount required to properly regenerate the resin in your softener.
#2. A standard forty pound bag of sodium chloride costs about eight dollars, while a standard forty pound bag of potassium chloride costs roughly forty dollars.
If you are considering making a switch, you can anticipate a five fold increase in your salt budget.
One of the major problems with sodium chloride water softeners is the fact during regenerations they require high strength brine, and a lot of that ends up going down a sanitary sewer or directly on the ground in a yard. When brine ends up going into a yard, it not only kills your grass but if that water has the ability to make its way into a nearby lake or water stream it will kill freshwater fish.
Additionally, if you are using shallow well water and your well is directly below your softener’s backwashing line you could potentially run into a situation where you are saturating your drinking water with sodium and effectively turning it into undrinkable salt water.
With potassium chloride you don’t have the same issue with killing the grass and plants in your yard, because potassium acts as a fertilizer for plants. However, if the runoff from backwashes makes it’s way into a lake or stream there could be a potential for algae bloom issues. Which could end up killing fish too.
No matter which salt you choose, make sure the backwash line from your softener is plumbed into a sanitary sewer drain line.
One of the most important aspects of a softeners overall performance, is going to be proper materials for installation being used, the right resin for your water source, the correct installation and last but not least the correct settings being applied for the softener system’s operation based on your incoming water quality and NOT just left at factory settings. If any of these boxes are not checked off or done incorrectly your experience can be ruined no matter what the intended use is for. With that being said, if you are not one hundred percent confident in your abilities, make sure to hire a reputable, licensed and insured company to professionally install your system.
© 2024 Filtered Water Guide. All rights reserved.